Elani, a symptomatic obstructive HCM patient, and her mom, were compensated for their time.
If you're still short of breath or feeling tired on your current oHCM medication, you deserve the possibility of symptom improvement. In clinical studies, CAMZYOS not only improved symptoms* but also improved the ability to be more active again.† Ask your cardiologist about CAMZYOS.
Clinical Study Results

CAMZYOS is being studied in the longest clinical study for a treatment of its kind.‡
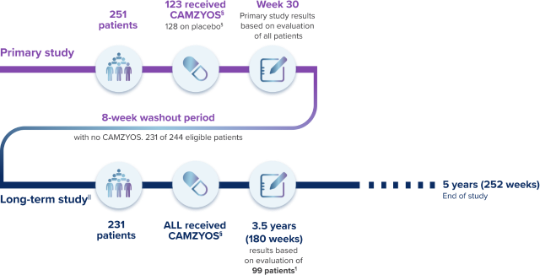
There are limitations to the long-term study. These data are not included in the CAMZYOS U.S. Full Prescribing Information. These data are based on real observations as part of an ongoing study. These data have not yet been tested to determine if results are based on real effect or random chance. All participants knew they were taking CAMZYOS, which may have influenced the results.
*Symptoms were measured using the New York Heart Association (NYHA) classification.
†The ability to be active was determined by measuring peak oxygen the body consumes during exercise. This was measured during exercise testing on a bicycle or treadmill.
‡CAMZYOS is a cardiac myosin inhibitor.
§Though it wasn't a requirement of the study, most of the participants remained on their previous medication, a beta blocker or calcium channel blocker.
||The primary goal of the long-term study was to evaluate safety and efficacy.
¶Amongst the efficacy outcomes, a range of 90 to 95 patients to date (of 231) have reached Week 180 for evaluation.
The study first looked at how many people experienced either:


Some improvement# in their ability to be active and an improvement in their symptoms

OR


More improvement# in their ability to be active and no worsening of their symptoms

Results showed that more people taking CAMZYOS achieved this outcome (37%, 45/123) compared with those taking placebo (17%, 22/128).
#Some improvement=improved pVO2 by at least 1.5 mL/kg/min. More improvement=improved pVO2 by at least 3.0 mL/kg/min.
What the data showed:
PRIMARY STUDY (30 weeks)

Approximately 2 out of 3 people taking CAMZYOS had their symptoms improve.
Of those taking CAMZYOS, 65% (80/123) improved by one or more NYHA class compared with 31% (40/128) of those taking a placebo.
LONG-TERM STUDY (180 weeks)

Approximately 3 out of 4 people (78%, or 74 out of 95 people)** taking CAMZYOS had their symptoms improve at approximately 3.5 years.
OF THOSE:
63
people (66.3%) had no symptoms or exercise limitations (NYHA class I)
29
people (30.5%) had mild symptoms and some exercise limitations (NYHA class II)
3
people (3.2%) had noticeable symptoms with minimal physical activity (NYHA class III)
**Amongst the efficacy outcomes, a range of 90 to 95 patients to date (of 231) have reached Week 180 for evaluation.
How was this measured? Symptom improvement was defined as a lower NYHA class at this time in the study. Symptomatic oHCM is a chronic disease and CAMZYOS is not a cure.
What the data showed:
PRIMARY STUDY (30 Weeks)

4x
more

decrease in obstruction to blood flow within the heart with CAMZYOS when compared to placebo.
People who took CAMZYOS had an average decrease in their LVOT gradient of 47 mmHg (started at 86 mmHg and went down to 38 mmHg), and people who took a placebo had an average decrease of 10 mmHg (started at 84 mmHg and went down to 73 mmHg).
LONG-TERM STUDY (180 Weeks)

79%

91 people taking CAMZYOS had an average reduction in LVOT gradient from 70 mmHg to 15 mmHg at approximately 3.5 years.
People who took CAMZYOS had an average decrease in their LVOT gradient of 55 mmHg (a 79% reduction) from the start of the study to the interim analysis at approximately 3.5 years.
How was this measured? At the beginning and end of the study, the obstruction in people’s hearts was measured with an echocardiogram after they exercised. This was done to find out how much blood was able to pump out of the heart in millimeters of mercury, or mmHg. Doctors call this measurement the left ventricular outflow tract, or LVOT, gradient. A lower LVOT gradient means there is less obstruction in the heart. Symptomatic oHCM is a chronic disease and CAMZYOS is not a cure. The data for LVOT gradient presented in the long-term study were measured while doing a breathing method called the Valsalva maneuver.
Primary study data


3x greater improvement in physical limitation and symptom burden

From when they started the study to when it ended, people taking CAMZYOS reported an average change in score of +14, and those who took placebo reported an average change in score of +4. The average baseline score was 71 for patients starting the trial.
How was this measured? How much people were bothered by their symptoms and experienced physical limitation was assessed using the Kansas City Cardiomyopathy Questionnaire (23-item version)—Clinical Summary Score (KCCQ-CSS). Higher scores represent better health status.
What the data showed:
PRIMARY STUDY (30 weeks)


3x greater improvement in shortness of breath

From when they started the study to when it ended, people taking CAMZYOS reported an average change in score of -3 and those who took placebo reported an average change in score of -1. The average baseline score was 5 for patients starting the trial.
LONG-TERM STUDY (180 weeks)


At approximately 3.5 years, improvement in shortness of breath for patients taking CAMZYOS was consistent to the primary study

From the beginning of the long-term study to this analysis, 90 people taking CAMZYOS reported an average change in score of -3.7. The average baseline score was 6 for people at the start of the study.
How was this measured? The frequency and severity of people’s shortness of breath was assessed using the Hypertrophic Cardiomyopathy Symptom Questionnaire—Shortness-of-Breath subscore (HCMSQ-SoB). Lower scores represent less shortness of breath.
CAMZYOS will not work for everyone. Individual results may vary.
Surgery Eligible

CAMZYOS reduced the number of patients who were eligible for, or who chose to undergo, surgery at 16 weeks (avoiding surgery)
A 16-week study of 112 people compared two groups of adults with symptomatic obstructive HCM. One group (56 people) took CAMZYOS while the other group (56 people) took a placebo. When the study started, everyone had symptoms. Some (7%) had moderate symptoms (NYHA Class II), but most (93%) had severe symptoms (NYHA Class III or IV). Everyone’s obstructive HCM was so advanced that they were eligible for heart surgery.
WHAT THE STUDY RESULTS SHOWED

The study looked at how many people either:
Remained eligible for surgery at Week 16
OR
Decided to proceed with surgery prior to or at Week 16
Results showed that CAMZYOS reduced the proportion of patients who were eligible for, or who chose to undergo, surgery. 18% of people taking CAMZYOS (~1 out of 5) and 77% of people taking placebo (~4 out of 5) remained eligible for surgery at 16 weeks or chose to undergo surgery prior to or at Week 16. Two people in each group decided to have surgery.


4 out of 5 people who took CAMZYOS experienced
improvements in their symptomatic obstructive HCM to the point that they were able to avoid surgery# at 16 weeks
vs 1 out of 5 people who took placebo

#No longer eligible and did not choose to undergo surgery at Week 16.
Most people in this study (95%) were also taking additional treatments for their HCM such as a beta blocker, calcium channel blocker, disopyramide, or a combination of these.
CAMZYOS will not work for everyone. Individual results may vary.
Side Effects

If you and your doctor are considering CAMZYOS, it’s important to know the answers to these questions:
What are the serious side effects of CAMZYOS?
A serious side effect is a side effect that can sometimes be life-threatening and lead to death.
CAMZYOS may cause serious side effects, including heart failure (a condition where the heart cannot pump with enough force).
In the long-term study, 10 (4.3%) people in the study experienced drug-related serious adverse events, which included cardiac failure (n=3), ejection fraction decreased (n=5), atrial fibrillation (n=1), and atrial flutter (n=1).
Get medical help right away if you experience new or worsening symptoms, including:
- Shortness of breath
- Chest pain
- Fatigue
- Racing heart (palpitations)
- Leg swelling
- Rapid weight gain
What are the most common side effects?
The most common side effects of CAMZYOS include dizziness and fainting (syncope).
In the long-term study, 228 (98.7%) people experienced ≥1 drug-related adverse reaction. The most common drug-related adverse reaction occurring in >5% of people were COVID-19 infection (n=92), dizziness, (n=41), hypertension (n=36), and nasopharyngitis (n=36).
- Tell your healthcare provider if you have any side effect that bothers you or that does not go away
- These are not all of the possible side effects of CAMZYOS
- Talk to your healthcare provider for more information about side effects. You may report side effects to the FDA at 1-800-FDA-1088. You may also report side effects to Bristol Myers Squibb at 1-800-721-5072
For more information, please see the U.S. Full Prescribing Information, including Boxed WARNING and Medication Guide for CAMZYOS. Talk to your healthcare provider for more information about this medication.
